Mind Controlled Bionic ‘Spinal Cord’ Could Help Paralyzed Patients Walk Using The Power Of Subconscious Thought
Scientists from Australia have developed a tiny paperclip sized device dubbed as “bionic spine,” that can be implanted into a blood vessel next to the brain to read electrical signals that lets wearers control an exoskeleton with just the power of though t. At present, it is going to be tested on three paraplegic patients in Melbourne in the following year, and will enable patients suffering from paralysis to control equipment that will subconsciously move robotic limbs.
“It’s the holy grail for research in bionics,” said Terence O’Brien, a doctor from the Royal Melbourne Hospital, according to the Telegraph.
Thomas Oxley, neurologist and lead researcher from the Royal Melbourne Hospital and the University of Melbourne said, “Our vision, through this device, is to return function and mobility to patients with complete paralysis by recording brain activity and converting the acquired signals into electrical commands, which in turn would lead to movement of the limbs through a mobility assist device like an exoskeleton. In essence this a bionic spinal cord.”
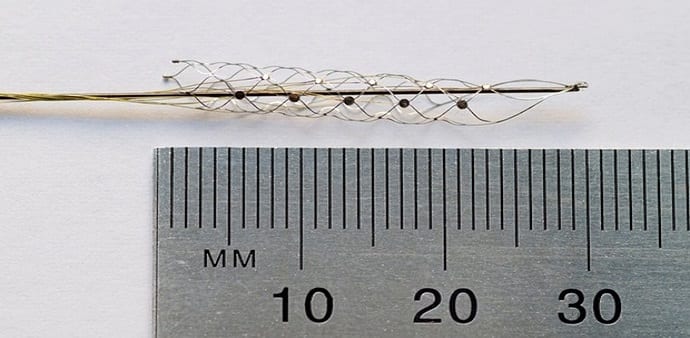
One of the biggest advantages of the new device – basically the size of a paperclip measuring 3 cm long and a few millimeters wide – is easy to implant. An experimental treatment uses electricity to help people suffering from paralysis stand up and even walk!
A team of 39 scientists implanted a 1-inch stent, with 12 electrodes beside the brain’s motor cortex that controls movement. This implantation involves the insertion of the stent into a neck vein through a catheter and then pushed up into the cortex.
According to the Sydney Morning Herald, due to the elastic properties of the metal stent, it can be bent and compressed, so that it easily traverses through the brain.
In just hours, it can reduce the risk of the brain injury that might accompany such implants.
The stent can get electrical signals sent out from the cortex and then to a device implanted in the shoulder. This then translates the signals into commands that might be sent wirelessly to bionic limbs or exoskeleton suits.
“The technical problem was how do you safely leave electrodes inside the brain, in a blood vessel inside the brain, without causing any damage to the subject,” said Oxley. “We have been able to create the world’s only minimally invasive device that is implanted into a blood vessel in the brain via a simple day procedure.”
“This is a procedure that Royal Melbourne staff do commonly to remove blood clots,” one of the team, Nicholas Opie from the University of Melbourne, told Melissa Davey at The Guardian. “The difference with our device is we have to put it in, and leave it in.”
The tiny electrodes on its exterior will stick to the walls of a vein once the bionic spine is implanted, and start recording electrical signals from the motor cortex. These signals are then transmitted to another device implanted in the patient’s shoulder, which translates them into commands to control wheelchairs, exoskeletons, prosthetic limbs, or computers via bluetooth.
This isn’t something patients will immediately know how to do. Patients will need a buffer time to use it, and along with some training will help them to take advantage of their thoughts to manoeuver bionic limbs and other apparatuses, which will eventually be controlled by their subconscious.
The “bionic spinal cord” could help many people, not just those suffering from paralysis, but those with epilepsy, Parkinson’s and other neurological disorders. And it could help injured vets, especially the tens of thousands in this country coming home with spinal cord injuries or without limbs, says neurophysiologist Professor Clive May.
While this is certainly not the first piece of technology designed to give paralyzed patients the ability to move again using neural signals, the team behind it says it’s an improvement on previous devices because of how tiny it is.
“[M]ost require invasive surgery involving removing a piece of the skull, known as a craniotomy, and which carries a risk of infection and other complications,” Davey explains for The Guardian, adding that a few recently unveiled devices involve bulky electrode caps and robotic suits.
“[A]nother existing procedure, which involves puncturing thousands of electrodes into the brain, is only effective for up to a year before the brain starts treating it as a foreign object and grows scar tissue over it,” she describes.
The team plans to start human trials in 2017, which so far has only been tested in sheep. The participants will be selected from the Royal Melbourne Hospital’s Austin Health, and will be the first humans to trial the device.
The technology has been described in Nature Biotechnology.