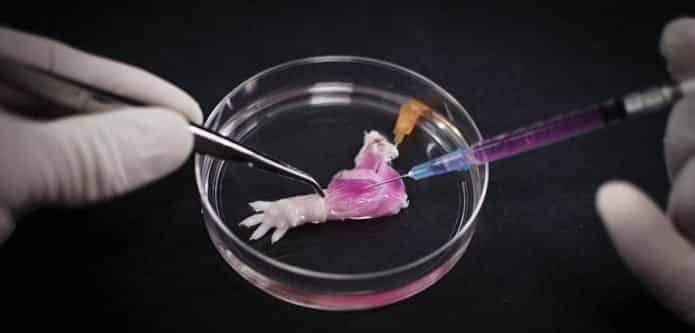
Researchers from the Massachusetts General Hospital produce a rat limb in lab for the first time
Harald Ott, a researcher and thoracic surgeon at Massachusetts General Hospital (MGH) spent weeks in a lab tending to a tiny rat’s forelimb. He got a special incubator for it, monitored it daily, cared for its every need. He is the proud parent of the world’s first lab-grown biolimb — a living, functioning, artificial leg that responds to stimuli and even circulates blood, the hospital announced Tuesday. Though it’s still a long way off from made-to-order transplants for humans, Ott and other regeneration experts say that the tiny pink rat leg is a step toward the future of artificial limbs.
“This is science fiction coming to life,” Daniel Weiss, a lung regeneration specialist at the University of Vermont College of Medicine, told the New Scientist. The secret to building a living, functioning, artificial limb starts with a dead one.
Ott and his team built the leg using a technique called decellularization, which involves stripping living cells from the limbs of dead donors (in this case, other rats) to expose their “scaffolds” — all of the inert, non-living parts. He then “recellularized” the limb by planting the cells that make up blood vessels and muscles onto the scaffolds, he told The Washington Post. Pop the whole thing in a specially designed bioreactor, allow it to grow for two weeks, graft some skin onto the fledgling leg, and the doctors had themselves their own, home-grown rat limb (minus the bones and cartilage). All they needed was a rat to attach it to.
Through the use of a special nutrient solution and regular doses of electrical stimulation, the scientists were able to successfully grow the new limb to a working state. The vascular cells were found to be functioning normally while the muscles contracted at 80 percent of the strength of the muscles found in newborn animals.
“We have shown that we can maintain the matrix of all of these tissues in their natural relationships to each other, that we can culture the entire construct over prolonged periods of time, and that we can repopulate the vascular system and musculature,” said Harald Ott MD, leader of the study, as CNET reports.
Ott, an assistant professor of surgery at Harvard Medical School, noted that the next challenge is regrowing nerves within a limb graft and reintegrating them into the recipient’s nervous system.
Researchers added that over 1.5 million Americans have lost a limb. While prosthetic technology has greatly advanced, there are still limits in function and appearance. Additionally, donor hand transplants expose recipients to the risks of life-long immunosuppressive therapy.
As you might expect, there’s still a lot of progress to be made before the same ideas can be transferred to growing a human limb, but the findings published in the journal Biomaterials are very motivating. For Ott and his team, the next goal is to repeat the procedure with a baboon before work begins on working out exactly how a lab-grown limb could be attached to a body with no adverse side effects.
There are all kinds of potential complications in trying to graft a living, functioning limb onto someone’s body – not least the reaction of the existing tissue – but as human hand transplants have proved, they’re not necessarily insurmountable. If scientists can make the process work then natural limb transplants would offer many advantages over the robotic prosthetics in use at the moment – the ability to feel heat and pressure, for example, plus a more natural learning process for the brain as it adjusts to the new appendage.
Ott predicted that doctors will be testing lab-grown human limbs in about a decade. One day, he thinks, people will be able to donate arms and legs the way they now donate kidneys. The old organs will be stripped of their soft tissue, seeded with new cells, and given to amputees, like a futuristic organ refurbishment program.